Chemical Bonding & Molecular Structure
SECTION 1 IONIC BOND OR ELECTROVALENT LINKAGE
An ionic bond is formed by the complete transfer of electron(s) from one atom to another. Atoms of metals generally lose electrons and those of non-metals gain electrons.
FORMATION OF SODIUM CHLORIDE, NaCl
A sodium atom (Z = 11) transfers its valence electron to a chlorine atom (Z = 17). The sodium atom by losing an electron acquires the electronic configuration of neon (1s22s22p6) and becomes sodium ion Na+ carrying a unit positive charge. The chlorine atom by gaining an electron acquires the stable configuration of argon (1s22s22p63s23p6) and becomes a chloride ion, Cl, with a unit negative charge.
The transfer of electron results in the formation of an ionic bond.
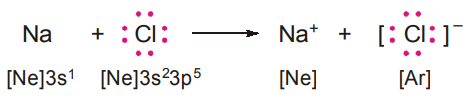
- Here we have used Lewis dot symbols in which the symbol of an element is surrounded by dots (or crosses) to represent electrons in the outermost (valence) shell. Formulae of compounds using Lewis symbols are called Lewis formulae.
- When atoms form a bond by electron transfer, the number of electrons lost and gained must be equal, because the resulting ionic compound is neutral.
- Loss of electron is called oxidation; thus, Na is oxidized to Na+. The gain of electron is reduction; thus, Cl is reduced to Cl–. Formation of an ionic bond from the elements is an oxidation-reduction or redox reaction. Generally, the metal is oxidised and the non-metal is reduced.
- Na+ and Ne are isoelectronic: since they contain the same number of electrons. Similarly, Cl– and Ar are isoelectronic.
- Because Na+ and Cl– carry opposite charges, electrostatic forces of attraction hold them together. Sodium chloride may be represented as Na+Cl–.
DETERMINATION OF LATTICE ENTHALPY
The direct calculation of lattice enthalpy is quite difficult because the required data is often not available. Therefore, lattice enthalpy is determined indirectly by the use of the Born-Haber cycle.
This cycle developed by Born and Haber, uses ionization enthalpies, electron gain enthalpies and other data for the calculation of lattice enthalpies. The procedure is based on the Hess’s law, which states that the enthalpy of a reaction is the same, whether it takes place in a single step or in more than one step.
The assumption is that the formation of an ionic compound may occur either by direct combination of the elements or by an alternate process in which following steps are involved. In both the cases energy involved is the same..
- The reactants are converted into gaseous state.
- The gaseous atoms are converted into ions.
- The gaseous ions are combined to form ionic compound.
For example, Born-Haber cycle, for the calculation of lattice enthalpy of simple alkali metal halide “MX” is shown here.

If we calculate ∆H5Θ from equation (i) and change its sign, we will get lattice enthalpy of MX(s). The following example illustrates the Born-Haber cycle.
SECTION 2 : COVALENT BOND
A covalent bond is formed by the sharing of a pair of electrons between two atoms, each atom contributing one electron to the shared pair. The shared pair of electrons should have opposite spins and they are localized between the two nuclei concerned. A covalent bond is usually represented by a short line (i.e., a dash) between the two atoms. Note that the covalent bond consists of a pair of electrons shared between two atoms, and occupying a combination of two stable orbitals, one of each atom; the shared electrons of each covalent bond are counted for each of the two atoms connected by the covalent bond.
The difference between the electronegativities of the combining atoms is less than 1.7.
SECTION 3 : VALENCE BOND THEORY OF COVALENT BONDING
3.1 SIGMA (σ) ΑND Pi (π) BONDS
Valence bond theory explains that a covalent bond is formed by the overlapping of the electron clouds of the atomic orbitals of the constituent atoms. The greater the overlap, the stronger the bond.
- Formation of Hydrogen Molecule
Hydrogen (1S1) has only one electron in its 1s-orbital. When two hydrogen atoms come together, overlap of their s-orbitals take place (s-s overlap), energy is released (bond energy) and a covalent bond called the σ bond is formed.
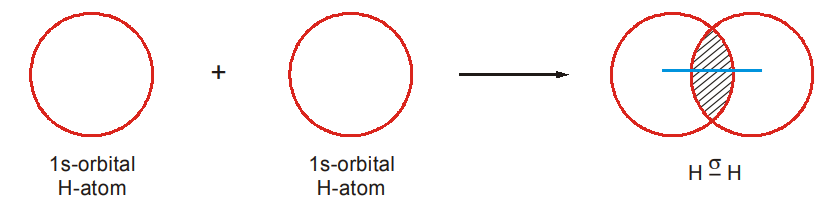
The electrons shared between the two atoms are to a large extent located in the region of space between the two nuclei. So, the region of overlap is the region of high electron density. The electron density (or electron cloud) is distributed symmetrically about the bond axis, i.e., the line joining the nuclei. Such a bond formed by the axial overlapping of the orbitals is called a sigma (σ) bond.
- Formation of Hydrogen Fluoride Molecule
H 1s1 F 1s22s22p5
The 1s-orbital of the hydrogen atom and one of the 2p-orbitals of the fluorine atom contain only one electron each. The 1s-orbital of the hydrogen atom and the partly filled p-orbital of the fluorine atom overlap axially and form a o bond (s-p overlap).
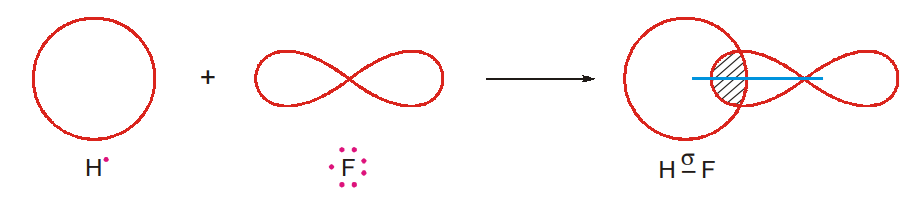
- Formation of Chlorine Molecule
Cl 1s22s22p63s23p5
One of the 3p-orbitals of the chlorine atom contain only one electron. The half-filled p-orbital of a chlorine atom overlaps axially with the half-filled p-orbital of the other chlorine atom and forms a σ-bond (p-p overlap).
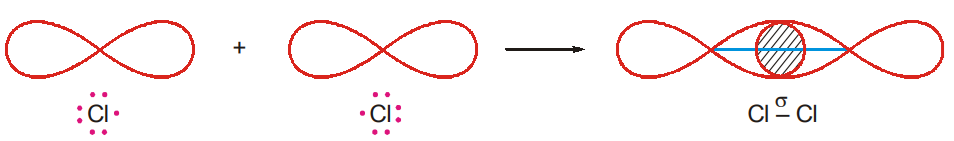
- Formation of Nitrogen Molecule
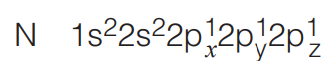
There are three unpaired electrons in the 2p-orbitals of a nitrogen atom. When two nitrogen atoms combine the three 2p-orbitals of one atom mutually overlap with those of the other atom and form three bonds.
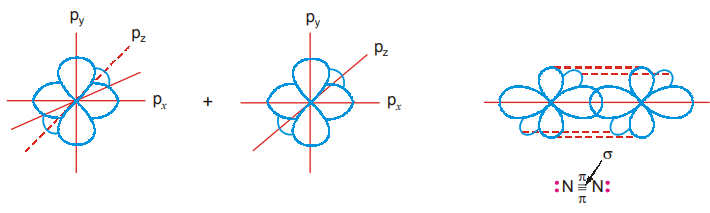
Suppose the orbitals approach along the x-axis the p-orbitals overlap axially and form a o-bond (p-p overlap). The py and pz-orbitals of the N atoms cannot overlap axially and so make a lateral (side to side) overlap forming two Pi (π) bonds.
Generally, in any multiple bond between two atoms one bond is a g-bond and the others π- bonds. A double bond will consist of a o-bond and a t-bond and a triple bond will consist of a o-bond and two r-bonds. In a π-bond formed between two p-orbitals, the upper lobe overlaps the upper lobe and the lower lobe overlaps the lower lobe. Together they constitute π-bond. The π-electron cloud will lie above and below the plane of the bond.
SECTION 7 : HYBRIDIZATION
- sp3 HYBRIDISATION
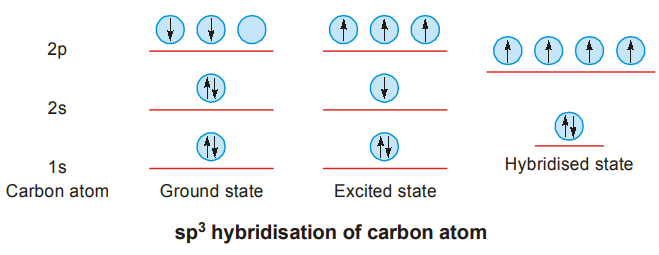
Carbon atom has the electronic configuration 1s22s22p1x2py1. It has two half-filled orbitals.
It should be expected to show a covalency of 2. In its millions of compounds carbon shows tetracovalency. To explain this the concept of hybridisation is introduced. Consider the formation of methane, CH4.
It may be supposed just for the sake of a picture that one of the electrons in the 2s-orbital is promoted to the vacant px, orbital (excited state). This is possible because energy released during bond formation will compensate for this. Then the four orbitals, one s and three p-orbitals, get mixed up and form four new ‘hybrid’ orbitals, of equal energy which are called sp3 hybrid orbitals, as they are formed by the mixing up (or blending) of one s and three p-orbitals.
So, hybridisation is nothing but combination of a certain number of atomic orbitals of slightly different energies to form the same number of new (hybrid) orbitals of equal energy.
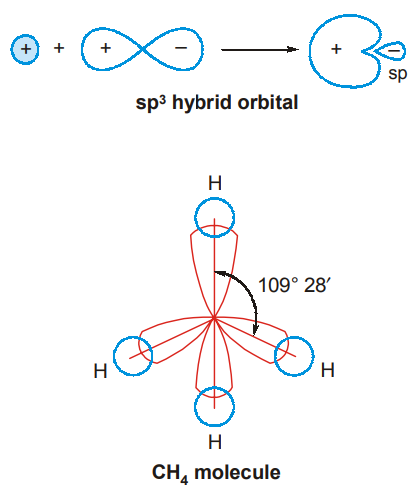
Each sp3 hybrid orbital is like a p-orbital, but with 2 lobes of unequal size (In figures, the small lobe is usually omitted).
Since the hybrid orbitals repel one another, they orient themselves with an angle of 109°28′ between them and point to the four corners of a regular tetrahedron. Each hybridized orbital overlaps the 1s-orbital of a hydrogen atom and forms a σ bond. Each sp3 hybridized orbital has one- fourth s-character and three-fourths p-character. Note that a hybrid atomic orbital from s and p-orbitals can form only σ bonds. (4C-H σ bonds.)
Formation of Ethane
In this case there is sp3-sp3 overlap resulting in the formation of the C-C bond and sp3-s overlap forming C-H bonds. (1 C-C σ bond and 6 C-Η σ bonds.)
- sp2 HYBRIDISATION
Formation of Ethylene Molecule
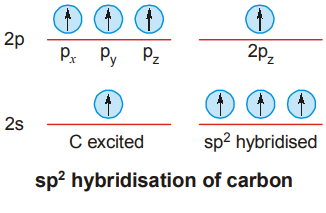
In the formation of ethylene, carbon atom undergoes sp2 hybridisation. Two of the sp2 orbitals of each atom form σ bonds with 1s-orbitals of hydrogen atoms by axial overlapping.
The sp2–sp2 overlap results in the formation of a C – C σ bond. The two carbon atoms and the four hydrogen atoms are all in the same plane and the bond angles are 120°.
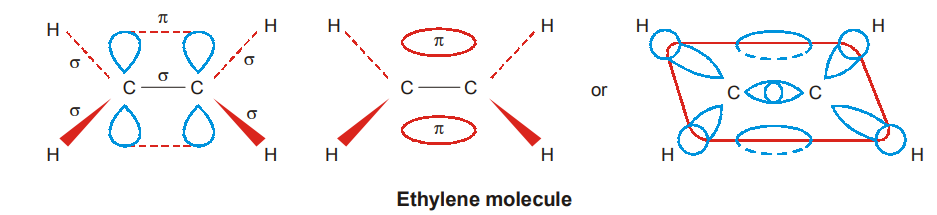
At right angles to this plane there remains the 2pz, orbital of each carbon atom which overlap laterally to form a π bond between the two carbon atoms. The double bond between the two carbon atoms consists of a σ bond and a π bond. (4 C – Η σ bonds, 1 C – σ bond and 1 π bond)
- sp-HYBRIDISATION
One s and one p-orbital combine to form two hybrid orbitals known as sp (or linear or diagonal) orbitals. They are of equal energy and are collinear. Each sp-orbital has one-half s character and one-half p character.
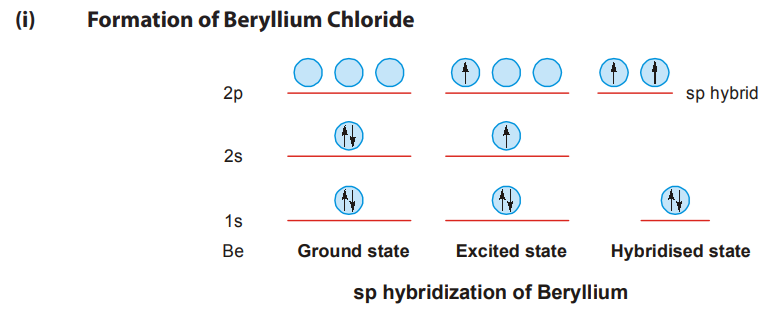
The sp hybrid orbitals of beryllium atom overlaps with the vacant px-orbitals of two chlorine atoms and form two σ bonds. Cl σ Be σ Cl since sp hybrid orbitals protrude along the axis farther than the corresponding s or p-orbitals they are able to overlap better and form stronger bonds than s or p-orbitals alone.
SECTION 8 : BOND LENGTHS, BOND ANGLES & BOND ENERGY
Bond length is defined as the distance between the nuclei of the two atoms constituting a chemical bond. It is expressed in Å.
Bond energy is the average energy released, when a covalent bond is formed by bringing two isolated, neutral, gaseous atoms from an infinite distance. It is expressed in kJ mol-1. The average energy required to break a particular bond in a molecule is its bond energy.
Note : When a C–H bond is broken in CH4 yielding CH3 and H, the energy required is bond dissociation energy. But 1/4 of the energy needed to break all the bonds in CH4 is the bond energy for C–H.
Covalent radii of elements which normally form covalent bonds are given in Table below. Bond length between two atoms can be calculated by adding the relevant covalent radii.
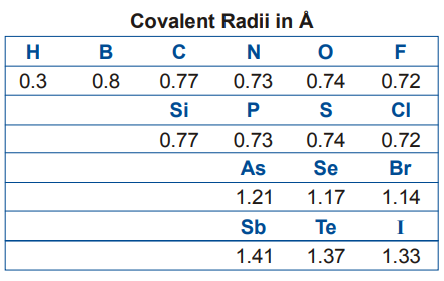
- BOND LENGTH AND BOND STRENGTH
The shorter the distance between a given pair of covalently bonded atoms, the greater the bond energy and hence the stronger the bond; e.g., the observed distances and energies of the bonds between two carbon atoms in ethane, ethylene and acetylene are:
| H3C – CH3 | H2C = CH2 | HC º CH | |
| Bond length (Å) | 1.54 | 1.33 | 1.2 |
| Bond energy (kJ mol–1) | 333.9 | 589.9 | 811.7 |
This shows that bond length decreases will increase in the number of bonds between the two given atoms. Multiple bonding results in greater electron density between the nuclei.
So, the nuclei are more strongly attracted by the increased electron density and are brought closer.
Multiple Bond Covalent Radii (in Å)
|
B = 0.76 |
C = 0.67 | N = 0.6 | O = 0.55 |
|
B º 0.68 |
C º 0.6 |
N º 0.6 |
O º 0.5 |
| Si = 1.07 |
S = 0.94 |
By comparing these values with those of single bond covalent radii, it will be seen that the double bond radius of an atom is about 13% and the triple bond radius is about 22% less than the corresponding single bond radius.
SECTION 9 : MOLECULAR GEOMETRY AND VSEPR (Valence Shell Electron Pair Repulsion Theory)
Molecular geometry is the three-dimensional arrangement of atoms in a molecule. A molecule’s geometry affects its physical and chemical properties such as melting point, boiling point and the types of reactions it undergoes.
Electron pairs in the valence shell (outermost electron-occupied shell of an atom) of an atom repel one another. In a polyatomic species, the repulsion between electrons in different bonding pairs causes them to remain as far as possible.
Thus, the geometry assumed by the species ultimately minimizes the repulsion. This approach is called valence-shell electron-pair repulsion (VSEPR) theory because it accounts for the geometric arrangements of electron pairs around a central atom in terms of the electrostatic repulsion between electron pairs.
- MOLECULES WITH CENTRAL ATOM HAVING ONE OR MORE LONE PAIRS
In such molecules, there are three types of repulsive interactions-between bonding pairs, between lone pairs and between a bonding pair and a lone pair. In general, according to VSEPR theory, the repulsive forces decrease in the following order: lone pair-lone pair repulsion > lone pair-bond pair repulsion > bond pair – bond pair repulsion.
Bond pair electrons are held by the attractive forces exerted by the nuclei of the two bonded atoms. These electrons have less “spatial distribution” than lone pairs, i.e., they take up less space than lone pair electrons, which are associated with only one nucleus (or one atom). Because lone-pair electrons in a molecule occupy more space, they experience greater repulsion from neighbouring lone pairs and bonding pairs.
To keep track of total number of bonding pairs and lone pairs, we designate molecules with lone pairs as ABxEy, where A is the central atom, B is the surrounding atoms and E is a lone pair on A. Both x and y are integers, x = 2, 3….. and y = 1, 2….. Thus, x and y denote the number of surrounding atoms and number of lone pairs on the central atom, respectively.
- Molecules with General Formula AB2E
Example of this type is SO2. The Lewis structure of SO2 is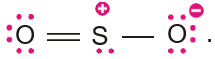
VSEPR theory treats double bond and triple bonds as though they were single bonds. Thus, SO2 molecule can be viewed as having three electron pairs on the central atoms, of which, two are bonding pairs and one is a lone pair. The overall arrangement of three electron pairs is trigonal planar. But since one of the electron pair is a lone pair, the SO2 molecule looks like :
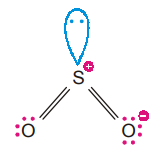
This shape is referred as bent or angular. The shape is determined only by the bonding pairs and not by lone pairs. Since lone pair repels the bonding pairs more strongly, the SO bonds are pushed together slightly and the OSO angle is less than 120°.
- Molecules having General Formula AB3E
The general formula AB3E is exhibited by the molecule NH3. Ammonia has overall four electron pairs, of which three are bonding pairs and one is lone pair. The overall arrangement of four electron pairs is tetrahedral but since one of the electron pairs is a lone pair, so the shape of NH3 is trigonal pyramidal.
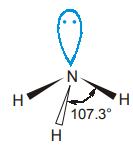
Because the lone pairs repel the bonding pairs more strongly, the three N-H bonds are pushed closer together. Thus, the HNH bond angle is smaller than the ideal tetrahedral angle of 109°48′.
- Molecules with General Formula AB2E2
Example of such a molecule is H2O. A water molecule has 2 bonding pairs and two lone pairs
The overall arrangement of the four electron pairs in water is tetrahedral. However, unlike NH3, H2O has 2 lone pairs on the central O atom. These lone pairs tend to be as far from each other as possible.
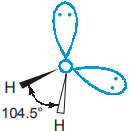
Consequently, the two OH bonding pairs are pushed toward each other and H2O shows even greater deviation from tetrahedral angle than in NH3. The shape of H2O is referred as bent or angular with HOH bond angle of 104.5°.
SECTION 12 : DIPOLE MOMENT
A dipole consists of a positive and an equal negative charge separated by a distance within a molecule. The degree of polarity of a bond is given by the dipole moment (µ), which is the product of either charge (e) and the distance (d) between them. µ = d × e. ‘e’ is of the order of magnitude of the electronic charge, i.e., about 10–10 esu and d is the distance between the atomic centres, i.e., about 10–8 cm. Hence, dipole moments may be expected to have values around 10–10 × 10–8 = 10–18 esu-cm. It is however, general practice to express dipole moments in Debye units (D), 1 D = 10–18 esu-cm.
If the charge is in SI units (Coulombs) and d in metre, µ will be coulomb-metre (C.m) units.
1D = 3.336 × 10–30 C.m
Any covalent bond which has a certain degree of polarity will have a corresponding dipole moment, though it does not follow that compound containing such bonds will have dipole moments, for the polarity of the molecule as a whole is the vector sum of the individual bond moments. For example, CO2 has zero dipole moment, although the C = O bond is a polar bond. This shows that CO2 is a linear molecule, O = C = O, so that the dipole moments of the two C = O bonds cancel out. The C → Cl bond has a definite polarity and a definite dipole moment but carbon tetrachloride has zero dipole moment because it is a tetrahedral molecule, and the resultant of the 4C – Cl bond moments is zero. On the contrary CH3Cl, CH2Cl2 and CHCl3 have definite dipole moments.
SECTION 13 : HYDROGEN BONDING
A hydrogen atom normally forms a single bond. In some compounds, however, the hydrogen atom may be located between two atoms acting as a bridge between them. Hydrogen atom is now involved in two bonds, one a normal covalent bond, the other a hydrogen bond. A hydrogen bond is always formed between two small, strongly electronegative atoms such as fluorine, oxygen and nitrogen.
- INTERMOLECULAR HYDROGEN BONDING-MOLECULAR ASSOCIATION
- Hydrogen Fluoride : From molecular measurements, it is known that hydrogen fluoride is associated (i.e., many molecules are joined together). HF is a polar molecule, with the fluorine atom acquiring a slight negative charge and the hydrogen atom acquiring an equal positive charge. The electrostatic attraction between the oppositely charged ends results in hydrogen bonding as shown below.
H – F … H – F … H – F …
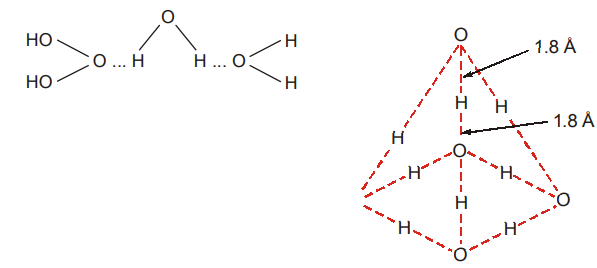
Many H – F units are held together, as (HF)n, by hydrogen bonding. The covalent H – F bond is much shorter than the F … H hydrogen bond; so a hydrogen bond is much weaker than a covalent bond. Fluorine, with the highest electronegativity forms the strongest hydrogen bond. The nature of the hydrogen bond is considerably electrostatic.
- Water : The high boiling point compared to that of hydrogen sulphide is due to molecular association through hydrogen bonding.
The crystal structure of ice shows a tetrahedral arrangement of water molecules. Each oxygen atom is surrounded tetrahedrally by 4 others.
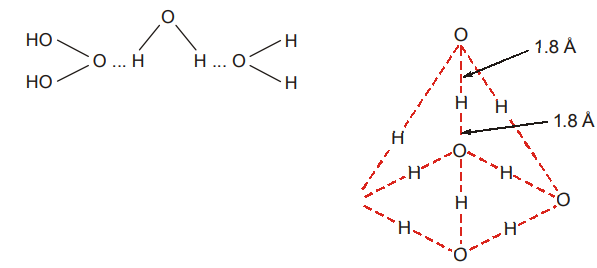
Hydrogen bonds link pairs of oxygen atoms together as shown below in figure. The arrangement of water molecules in ice is a very open structure and this explains the low density of ice. When ice melts, the structure breaks down and the molecules pack more closely together so that water has a higher density; this packing goes to a maximum upto a temperature of 4 C.
- INTRAMOLECULAR HYDROGEN BONDING
Sometimes hydrogen bonding may take place within a molecule; this is known as intramolecular (or internal) hydrogen bonding. It may lead to the linkage of two groups to form a ring; such an effect is known as chelation, in the case of complex compounds.
- Nitrophenols :
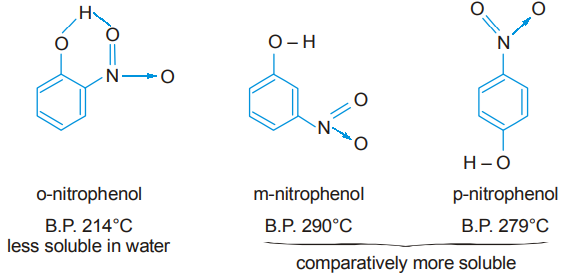
Because of the proximity of –OH and –NO2 groups in o-nitrophenol there is intramolecular hydrogen bonding which prevents intermolecular hydrogen bonding between two or more molecules. Since molecular association cannot take place, the boiling point of o-nitrophenol is lower than that of the other two. Because of the distance between –OH and –NO2 groups in m- and p-nitrophenols there is no possibility of intramolecular hydrogen bonding. Intermolecular hydrogen bonding may take place to a certain extent which causes some degree of molecular association; this is responsible for the higher boiling points of the two nitrophenols.
Further, the formation of intramolecular hydrogen bonding in o-nitrophenol prevents it from entering into intermolecular hydrogen bonding with water and this explains its reduced solubility.
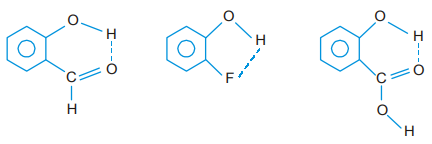
- Other molecules in which intramolecular hydrogen bonding is present are o-hydroxybenzaldehyde, o-fluorophenol and o-hydroxybenzoic acid.
SECTION 14 : MOLECULAR ORBITAL THEORY
The Valence Bond Theory (V.B. Theory) with the concepts of hybridization and resonance is used to explain the structure and properties of several molecules, but there are limitations. For example, the V.B. theory in its original form, is not able to explain the paramagnetic behavior of O2 molecule. Hence, the Molecular Orbital Theory (or M.O. Theory) given by Hund and Mulliken was developed. The following are the essential features of the M.O. Theory.
- In the M.O. model, all the electrons are taken together and considered as moving in the field of all the nuclei. (In the B. model, only the bonding electrons are considered and they are taken to move in the field of the nuclei involved in bonding.)
- The atomic orbitals are combined to form, what are called molecular orbitals and electrons are fed into these (In the V.B. model, electrons are fed into the atomic orbitals, which are then supposed to overlap.)
- The number of combining atomic orbitals is equal to the number of molecular orbitals
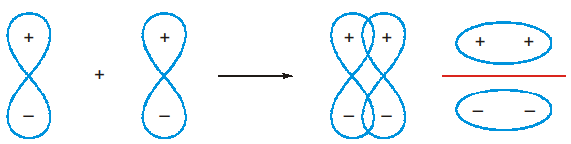
- When two atomic orbitals combine, two O’s are formed, of which one has a lower energy, while the other has a higher energy. The former is known as the bonding orbital and the latter antibonding. Mathematically, if ψ1 represents the wave function corresponding to orbital 1 and ψ2 for orbital 2, the total function is a linear combination of ψ1 and ψ2, i.e., ψ = ψ1 ± ψ2 (omitting the constants). This is known as linear combination of atomic orbitals (L.C.A.O.). Of these, ψ1 + ψ2 corresponds to the bonding M.O., while ψ1 – ψ2 corresponds to antibonding M.O., i.e., ψb = ψ1 + ψ2 and ψa = ψ1 – ψ2. The electron density or probability of finding an electron is directly proportional to ψ2.

- There are different notations for representing bonding and anti-boding O’s obtained from A.O’s. We give a simple notation below.
We have assumed the two pz orbitals to overlap end to end, so that the M.O. formed is of the ‘σ’ type (similar to the ‘σ’ bond in V.B. theory); then the two px atomic orbitals, as also the two py orbitals will overlap laterally to give M.O’s of the π-type. Some authors follow the convention of choosing two px orbitals for end to end (i.e., axial) overlap, so that the M.O’s formed are σ(px) and σ∗(px).Atomic orbitals that are mixed
s and s
pz and pz
px and px
py and py
Bonding M.O.
ss
s(pz)
p(px)
p(py)
Antibonding M.O.
σs∗
s*(pz)
p*(px)
p*(py)
- When electrons are successively placed in the M.O’s, Aufbau principle, Hund’s rule and Pauli’s principle are followed, as in the case of the atomic orbitals. Aufbau Principle : M.O’s are occupied in the order of increasing energy. The following is the general arrangement of M.O’s in the order of increasing energy. σ(1s) < σ∗(1s) < σ(2s) < σ∗(2s) < σ(2pz) < π(2px) = π(2py) < π∗(2px) = π∗(2py) < σ∗(2pz) … etc. The above is only a general order and slight variations often occur due to interaction between s and p orbitals. For example, sometimes, π(2px) = π(2py) < σ(2pz).
- Hund’s Rule of Maximum Multiplicity : The degenerate M.O’s are occupied singly, before any pairing could occur. The maximum capacity for each M.O. is 2 electrons.
- Only atomic orbitals of equal or nearly equal energies combine to give the O’s. In the case of homonuclear diatomic molecules, energies of corresponding A.O’s of the two atoms are equal. So, the above condition of combination of A.O’s assumes special significance in the case of heteronuclear diatomic molecules and it has to be used with caution.
- It has been already pointed out that electrons in the bonding O’s tend to pull the nuclei together and that the electrons in the antibonding M.O’s tend to separate them. Hence the combined influence of bonding and antibonding electrons may either stabilize or destabilize the molecule, depending on the relative number of these two types of electrons. The stabilizing power is expressed in terms of what is called the “bond order”.

The greater the bond order, the greater the bond stability and the shorter the bond distance.