Chemical Equilibrium
CHARACTERISTICS OF EQUILIBRIUM STATE
- Chemical equilibrium is dynamic in nature.
- At constant temperature, certain properties such as pressure, concentration, density or colour become constant.
- Equilibrium can be attained from either side, i.e., from the side of reactants or products.

- Catalyst does not change the equilibrium state but helps in attaining it rapidly.
LAW OF MASS ACTION
The law of mass action (given by Guldberg and Waage) states that the rate of a chemical reaction is proportional to the product of effective concentrations (active masses) of the reacting species, each raised to a power that is equal to the corresponding stoichiometric number of the substance appearing in the chemical reaction.
By the rate of a chemical reaction, we mean the amount of reactant transformed into products in unit time. It is represented by dx/dt.
Active mass means the molar concentration, i.e., the number of moles in 1 litre. Suppose 3 moles of nitrogen are present in a 4-litre vessel, the active mass of nitrogen = 3/4= 0.75 mole/litre. Active mass of a substance is represented by writing molar concentration in square brackets.
Active mass of reactant α molarityActive mass of reactant = γ × molarity
where γ is the activity coefficient.
∴ Active Mass = γ × molarity
For very dilute solutions, the value of activity coefficient is unity.
Active Mass can be taken to be same as Molarity.
EQUILIBRIUM CONSTANT KC AND KP
Let us have an equilibrium reaction as :
![]()
For this reaction, which is in equilibrium, there exist an equilibrium constant (Keq ) represented as :
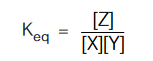
For the given equilibrium, irrespective of the reacting species (i.e, either X + Y or Z or X + Z or Y + Z or X + Y + Z) and their amount we start with, the ratio, 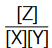 is always constant at a given temperature. This really looks amazing. Isn’t it? Let us see, how such a thing is possible.
is always constant at a given temperature. This really looks amazing. Isn’t it? Let us see, how such a thing is possible.
We have learnt that at the equilibrium, rate of forward and reverse reactions are equal and we also know the law of mass action. Using this, we can write
Rate of forward reaction α [X] [Y]
Rate of forward reaction = kf [X] [Y]
where kf is the rate constant for the forward reaction.
Similarly, rate of reverse reaction α [Z]
Rate of reverse reaction = kr[Z]
where kr is the rate constant for the reverse reaction.
At equilibrium,
Rate of forward reaction = Rate of reverse reaction.
∴ k f [X] [Y] = k r[Z]
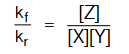
Since, kf and kr are constants at a given temperature, so their ratio 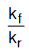 would also be a constant, referred as Keq.
would also be a constant, referred as Keq.

As Keq is the ratio of rate constants for forward and reverse reaction, so the value of Keq would always be a constant and will not depend on the species we have started with and their initial concentrations.
The given expression involves all variable terms (variable term means the concentration of the involved species changes from the start of the reaction to the stage when equilibrium is reached), so the ratio 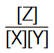 can also be referred as KC .
can also be referred as KC .

PROPERTIES OF EQUILIBRIUM CONSTANT
- It does not depend on initial concentration, because product will be formed in more amount when concentration of the ractants is increased, since the value of K remains constant.
- The value of K is constant at a fixed temperature but on increasing the temperature, value of K will change because concentration of reactants and products undergoing chemical reaction increases on increasing temperature.
- When the value of ‘KC’ is more than 1.0 then product is formed in greater amount, i.e., forward reaction is faster but when it is less than 1.0 the product is formed in lower amount, i.e., backward reaction is faster.
- Value of KC remains unaffected by the presence of catalyst, because catalyst has equal effect on the rates of forward and backward reactions.
- If the same reaction is brought to equilibrium by two different methods the values of equilibrium constant will be different under the two conditions.
- Some mathematical operations that can change the value of equilibrium constant are as given below :
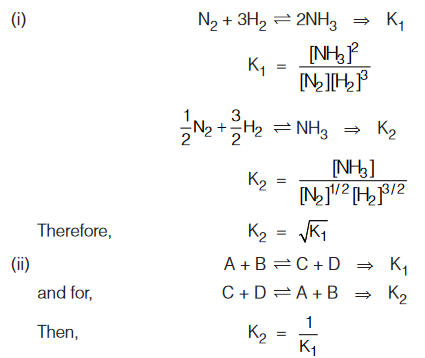
- The equilibrium constants Kc and Kp can be related as
KP = KC (RT)∆n
where ∆n = sum of the number of moles of gaseous products – sum of the number of moles of gaseous reactants.
R = gas constant
and T = absolute or Kelvin temperature at which equilibrium is established.
Since, partial pressures are generally noted in atm and concentrations are measured in 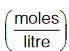 so the value of R used in the given expression should be in litre-atm per mole per Kelvin.
so the value of R used in the given expression should be in litre-atm per mole per Kelvin.
UNITS OF KC AND KP
Although it is not customary to mention the units of equilibrium constants KP and KC but when required, the unit of KC would be 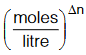 as the concentration of a species is generally expressed in moles/litre and the unit of KP
as the concentration of a species is generally expressed in moles/litre and the unit of KP
would be (atm)∆n as the partial pressure is generally measured in atm.
SECTION 6 : LE CHATELIER’S PRINCIPLE
It states that if a change is done to a system in equilibrium, the equilibrium condition is altered. A net reaction occurs in that direction which tends to relieve the external stress and finally a new equilibrium is attained.
To understand its application to a system, let us consider following example :
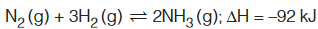 for forward reaction, ∆H = +92 kJ for backward reaction.
for forward reaction, ∆H = +92 kJ for backward reaction.
Note that in above reaction,
- Forward reaction is exothermic (towards formation of NH3) and backward reaction is endothermic (favours decomposition of NH3).
- Formation of NH3 result in decrease in number of moles, i.e., decrease in volume to right.
- Both reactants and products are gases, i.e., they will be influenced by changes in P, T and changing concentration.
EFFECT OF TEMPERATURE
Temperature can be increased by adding heat and can be decreased by taking out heat from the system. Increase or decrease in temperature reaction either shifts in forward or backward direction and it also changes the equilibrium constant.
Endothermic Reaction- Increase in temperature shifts the reaction in the forward direction.
- Decrease in temperature shifts the reaction in the backward direction.
- Increase in temperature, shifts the reaction in the backward direction.
- Decrease in temperature, shifts the reaction in the forward direction.
Effect of temperature on equilibrium constant by using van’t Hoff equation.
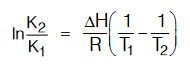
For Endothermic Reaction :
If T2 > T1 then K2< > K1
For exothermic reaction if T2 > T1 then K2 < K1.
SECTION 7 : THERMODYNAMICS OF EQUILIBRIUM CONSTANT
Although the equilibrium constant is not dependent on rate, but it is directly related to Gibb’s Energy (∆G). The relationship between Keq and ∆G is well established.
- If ∆G < 0, then the reaction is spontaneous and proceeds forward.
- If ∆G > 0, then the reaction is non-spontaneous.
- If ∆G = 0, then the reaction has achieved equilibrium. At this state no free energy is available to run the reaction.
Mathematically, for a reaction we should know that
∆G = ∆Go + 2.303RT log Q
Here, ∆Go is standard free energy change and Q is the reaction quotient of the reaction not in equilibrium. When equilibrium is achieved ∆G = 0 and Q = KC
So, 0 = ∆Go + 2.303RT log KC is what we get
⇒ ∆Go = –2.303RT log KC = –RT ln KC
In other words,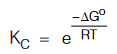
This equation can be used to determine the spontaneity of a reaction that is if ∆G o < 0 then e–∆Go/RT > 1 making KC > 1 which implies a spontaneous forward reaction.
If ∆Go < 0 then e–∆Go/RT < 1, i.e., K < 1 which implies a backward reaction spontaneity. Such reaction proceed forward to such a small extent that a very small amount of product is formed.
Relationship of ∆G° to the equilibrium constant K.
∆G for a reaction under any set of conditions is related to its value for standard conditions, that is, ∆G° by equation.
∆G = ∆G° + 2.303 RT log Q
Here, Q refers to reaction quotient of the reaction that is not in equilibrium.
Under equilibrium condition
Q = Kp = Kc = K
∆G = 0
∆G° = –2.303 RT log K
K is called thermodynamic equilibrium constant.