Modern Periodic Table Laws
Mendeleev’s Periodic Table
Periodic Law
“The physical and chemical properties of elements are a periodic function of their atomic masses.”
On the basis of this law he arranged all the elements in order of increasing atomic mass and he found that after certain regular intervals repetition in properties occur.
-
Merits of Mendeleev’s Table
- First systematic classification of elements by forming a table. Mendeleev’s periodic table has 8 groups and 7 periods.
- Correction of Atomic Masses of the elements was done.
- He left a certain gap in the periodic table for undiscovered elements and he predicted the properties of those elements correctly. For instance, what he called Eka-Aluminium is now a days Gallium and what he called Eka-Silicon is now a days Germanium.
-
Demerits of Mendeleev’s Table
- Anomalous position of Hydrogen. He failed to justify the position of Hydrogen because some properties of hydrogen were similar to alkali metals and some other properties were similar to halogens.
- Cause of periodicity: He failed to explain why the repetition in properties occur after certain regular intervals.
- He failed to accommodate Lanthanides & Actinides in the main body of the periodic table.
- Some pairs did not obey the rule of increasing atomic masses. For instance Tellurium with atomic number 52 had a mass of 127.60u but lodine with atomic number 53 had a mass of 126.90 which was less than that of Tellurium.
- He failed to justify the position of isotopes in the periodic table.
Modern Periodic Law And Modern Periodic Table
where Z is the atomic number of the target element and (a) and (b) are Moseley’s constants.
- The results of this experiment led to the re-framing of the periodic law. The modern periodic lav was hence propounded as, “The physical and chemical properties of elements are a periodic functions of their atomic numbers”.
Long Form of the Modern Periodic Table :
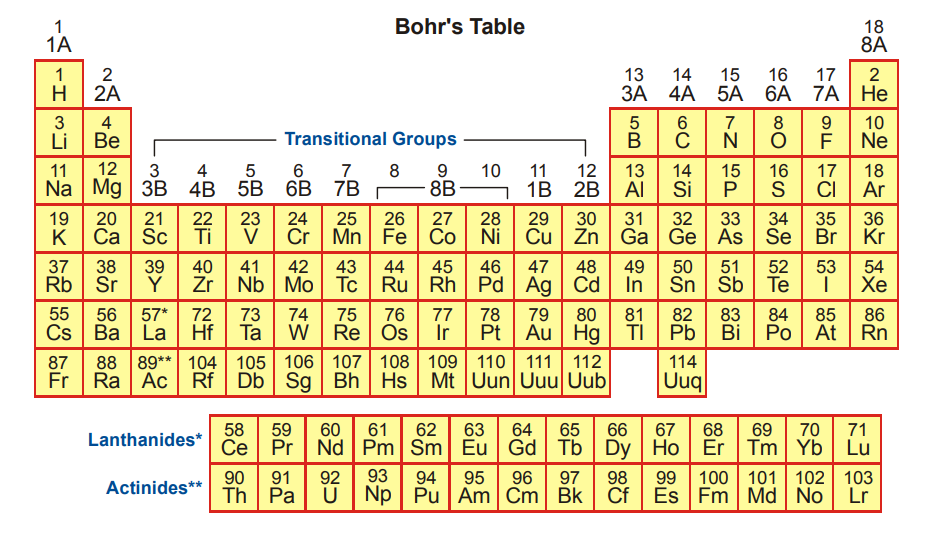
Some Important Points about the Modern Periodic Table :
- It consist of eighteen groups. Each group consists of a number of elements having the same outer electronic configuration.
- The elements of 18th group are called inert gases or noble gases.
- The elements of groups 3, 4, 5, 6, 7, 8, 9, 10, 11 and 12 are called transition elements.
- Two series each of 14 elements are placed at the bottom of the periodic table, known as Lanthanides and Actinides (Inner Transition Elements)
- There are 7 known periods (horizontal rows) in this table. Distribution of element in different periods are as below:

Atomic Size
- Atomic Radius: It is the distance from the centre of the nucleus to the outer most shell containing electron. It is impossible to isolate the atom and determine its radius precisely because its radius on is affected by its association with its neighbourhood. On the basis of the bond formed between atoms we can estimate the radius of the atom with great precision. Some of the radii are mentioned here:
- Ionic Radius: The lonic radius may be defined as the effective distance from the centre of the nucleus of the ion upto which it exerts its influence on the electron cloud.
-
-
- The radius of cation is always smaller than that of its parent atom due to higher effective nuclear charge after removal of electron.
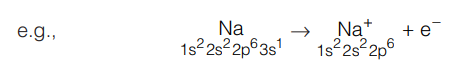 Due to removal of a valence electron, the number of electrons decrease. Each electron now experiences greater nuclear pull. As a result, the size of cation is smaller than that of the parent atom.
Due to removal of a valence electron, the number of electrons decrease. Each electron now experiences greater nuclear pull. As a result, the size of cation is smaller than that of the parent atom. - The radius of anion is always larger than that of its parent atom.
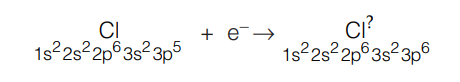 An anion is formed by gain of electron, which increases the number of electrons in the same shell. The effective nuclear charge now decreases and each electron experiences reduced nuclear pull. Repulsions between valence electrons now cause the cloud to expand making the anion larger in size than the neutral atom.
An anion is formed by gain of electron, which increases the number of electrons in the same shell. The effective nuclear charge now decreases and each electron experiences reduced nuclear pull. Repulsions between valence electrons now cause the cloud to expand making the anion larger in size than the neutral atom.
- The radius of cation is always smaller than that of its parent atom due to higher effective nuclear charge after removal of electron.
-
- Iso-electronicions: lons of different elements which have the same number of electrons but different magnitude of nuclear charge are called isoelectronic ions.
- Variation of size: The ionic radius of iso-electronic ions decreases with the increase in the magnitude of nuclear charge. e.g., among isoelectronic ions of the second period the order of radius should be C4> N3-> 02-> F-> Na+ > Mg2+ > Al3+ and among those of the third period: Si4-> P3-> S2-> CI > K+ > Ca2+
- Factors affecting the Atomic Radii :
- Number of Shell: More the number of the shells filled with electrons, larger will be size.
- Nuclear Charge: Nuclear charge attracts the electrons toward itself and attempts to decrease the size. This is nuclear charge effect. Generally, across the period size decreases because with increase in atomic number effective nuclear charge increases. As electrons are filled in the same shell they contract towards the nucleus causing size to decrease. Down the group size generally increases, addition of an extra shell causes effective nuclear charge to decrease on the valence electron.
- Screening Effect or Shielding Effect: The inner layer of the electron act as shield between nucleus and valence electron. This is known as shielding effect or screening effect as discussed earlier. Effective screening therefore attempts to increase the size and ineffective screening causes a decrease in atomic radius.
- Periodicity in Atomic Radius
- In a Period: The number of orbit remains same on going from left to right in a period while effective nuclear charge increases. So, atomic radius decreases across the period.
- In a Group: The atomic radius increases on going down a group due to addition of an extra shell.
Ionization Energy (Enthalpy)
It is the amount of energy required to remove the outer most electron from an isolated atom in gaseous state.
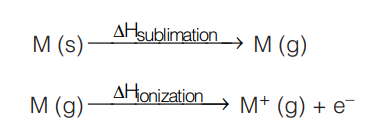
Neutral isolated gaseous atom is produced by firstly subliming the metal atom from its solid state. Further on, the energy needed to knock off the first outermost electron is called the first ionization enthalpy. As this is the energy required, so value of ionization enthalpy is always positive.
Unit wise, ionization enthalpy is expressed in kJ/mole or kCal/mol, however for an atom if the energy unit is in terms of eV then ionization enthalpy can also be refrred to as lonisation potential.
Successive lonisation Energy
- lonisation Energy (First): It is the amount of energy required to remove the outer most e from an atom in gaseous state.
- lonisation Energy (Second): It is the amount of energy required to remove the 2nd e- from same atom in gaseous state.
Important Fact: I.E2 > I.E1
Because 2nd electron has to be removed from a cation which has high effective nuclear charge and smaller size than the neutral atom.
Ionisation energy generally increases across a period and decreases down the group.
Successive ionization energies are always larger. There is no exception to this rule.
Electron Gain Enthalpy
It is the enthalpy change when an electron is added to the gaseous neutral atom.
Electron gain enthalpy provides a measure of the ease with which an atom adds an electron to form anion.
X(g) + e– → X–(g); ∆H = AegH ..(i)
The negative of the enthalpy change for the process shown in equation is defined as electron affinity of the atom undergoing the change for formation of anion.
Depending on the element, enthalpy change in process may be endothermic or exothermic.
When electron is added to gaseous neutral atom that has a natural tendency to accept an electron then energy is released, electron gain enthalpy is negative and electron affinity is positive.
When an electron is added to an element that does not have a natural tendency to accept an electron then energy will be absorbed electron gain enthalpy will be positive and electron affinity will be negative.
Electronegativity
Electronegativity is a measure of the tendency of an element to attract bonded electron pair towards (itself) in a covalent bonded molecule.
- Factors on which electronegativity depend:
- Atomic Size: Electronegativity is inversely proportional to the size. Small sized elements generally have higher electronegativity.
- Effective Nuclear Charge: Electronegativity is directly proportional to the effective nuclear charge. Elements such as fluorine have highest effective nuclear charge in the second period and hence have the highest electronegativity.
- Hybridization: More is the s-character in hybridization, higher will be electronegativity. The order of electronegativity among different hybridizations is: sp hybrid carbon >sp2 hybrid carbon > sp3 hybrid carbon
- Electronegativity Scales:
- Mulliken Scale: According to Mulliken,
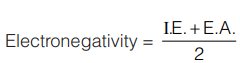 Both ionization energy and electron affinity are taken in eV/atom.
Both ionization energy and electron affinity are taken in eV/atom. - Pauling Scale: Linus Pauling developed a method for the calculation of relative electronegativity of element.
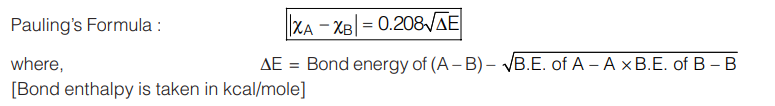 Relation between Mulliken and Pauling Scale: The value of electronegativity for an element in Mulliken scale is 2.8 times higher than Pauling’s value.
Relation between Mulliken and Pauling Scale: The value of electronegativity for an element in Mulliken scale is 2.8 times higher than Pauling’s value. - Allred Rochow’s Electronegativity: Allred and Rochow defined electronegativity as the force exerted by the nucleus of an atom on its valence electrons:
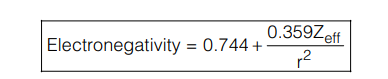 Here, Zeff is effective nuclear charge and r is the radius of the atom.
Here, Zeff is effective nuclear charge and r is the radius of the atom.
On the Pauling Scale: Fluorine is most electronegative element with an electronegativity value of 4.0 followed by oxygen with a value of 3.5.