Some Basic Concepts of Chemistry
SECTION 1 : LAWS OF CHEMICAL COMBINATION
1.1 THE LAW OF CONSERVATION OF MASS (LAVOISER, 1744) :
This law states “matter can neither be nor be destroyed or in a chemical reaction, the mass of the reactants is equal to the mass of the products”. The exception to this law is nuclear reactions where Einstein equation is applicable.
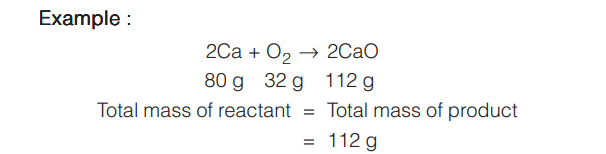
For a complete irreversible reaction total mass of reactants before reaction in equal to the total mass of products after reaction.
1.2 THE LAW OF CONSTANT COMPOSITION OR DEFINITE PROPORTION (PROUST, 1799)
This law states that “All pure sample of the same chemical compound contain the same elements combined in the same proportion by mass irrespective of the method of preparation”.
Example: Example: Differ Example: ent samples of carbon dioxide contain carbon and oxygen in the ratio of 3 : 8 by mass.
Similarly in water ratio of weight of hydrogen to oxygen is 1 : 8.
1.3 LAW OF MULTIPLE PROPORTION (DALTON, 1803)
“When the two elements combine to form two or more chemical compounds, then the weight of one of the elements which combines with a fixed weight of the other, bear a simple whole number ratio to one another. This is called the law of multiple proportions.
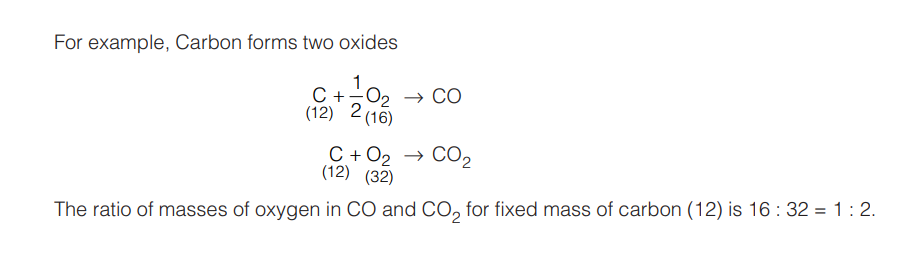
SECTION 2 : ATOMIC MASS AND MOLECULAR MASS
2.1 ATOMIC MASS
As atoms are very tiny particles, their absolute masses are difficult to measure. However it is possible to determine the relative masses of different atoms if small unit of mass is taken as standard (previously, this standard was mass of one atom of hydrogen and taken as unity). Later on, 1/16th mass of oxygen atom and now it is 1/12th mass of C-12 atom.
The atomic mass of an element can be defined as the number which indicates how many times the mass of one atom of the element is heavier in comparison to 1/12th mass of an atom of C-12.
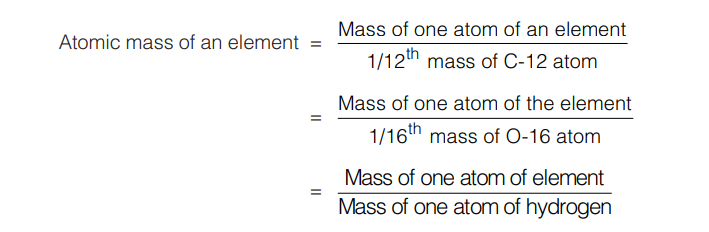
2.2 ATOMIC MASS UNIT
The quantity 1/12th mass of an atom of carbon-12 is known as atomic mass unit and is abbreviated as amu. The actual mass of one atom of carbon-12 is 1.9924 × 10–23 g or 1.9924 ×10–26 kg
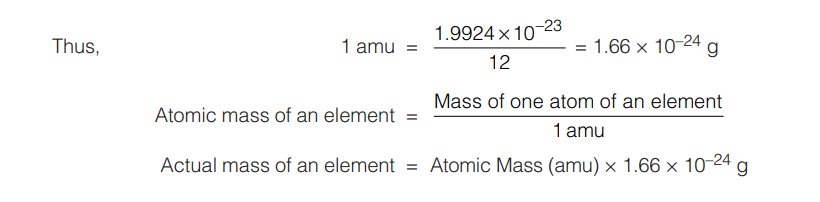
SECTION 4 : MOLE CONCEPT
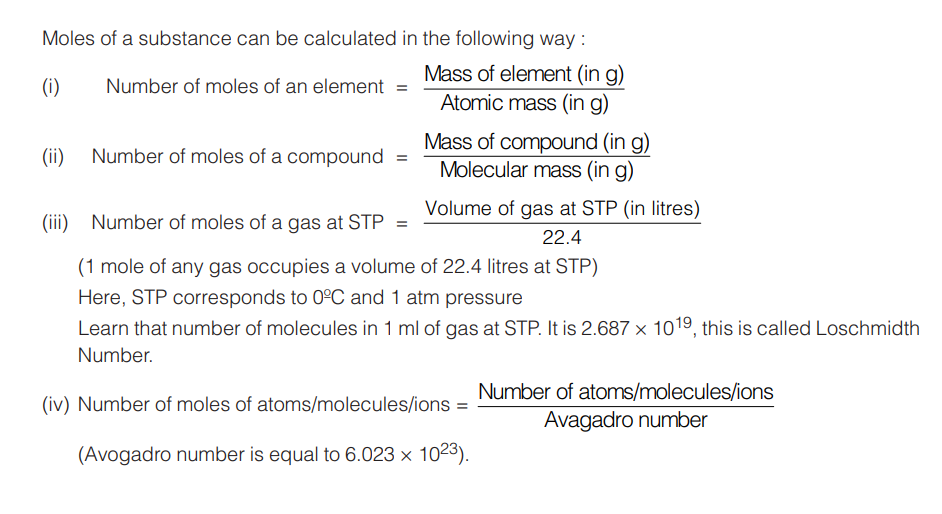
Mole is a collection of 6.023 × 1023 particles (atoms or molecules or ions). It is the SI unit for amount of a substance also. For instance, one mole CaO molecules refer to 6.023 × 1023 molecules of CaO.
SECTION 5 : MOLE CONCEPT AND GASES
It is imperative to know that gases can be quantified similar to solids and liquids. Many of the earlier developments related moles to gases and their volumes. To understand this clearly, we must learn that we presume all gases present in the universe to be ideal and for all ideal gases the Ideal Gas Equation holds true. This is given as: P × V = nRT. Here, P is the pressure of the gas, V is the volume of the gas (which is taken T to be the volume of the vessel in which it is contained), n are the number of moles of the gas and T is temeprature of the gas (in Kelvin). Here, R is the universal gas constant and its value can be taken to be 0.0821 l.atm/mol.K or 8.314 J/mol.K.
Some of the developments are listed here for your knowledge:
5.4 VAPOUR DENSITY
t is defined as a number which indicates how dense a gas is with respect to density of H2 gas at STP. Learn, that vapour density is just a number and hence dimensionless.
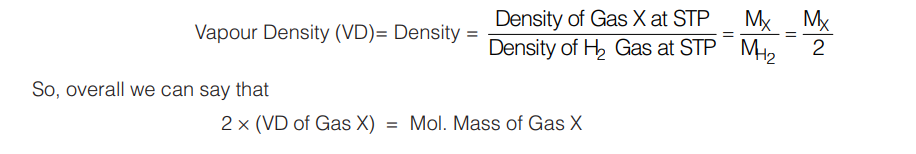
5.5 MOLAR VOLUME OF AN IDEAL GAS AT STP
We know from our earlier discussion that Standard Pressure for ideal gases is taken to be 1 atm and Standard Temperature is taken to be 273.15 K or 0°C. Under these conditions the volume of one mole of a ideal gas also called Molar Volume will be calculated as

5.6 GAY LUSSAC’S LAW OF COMBINING VOLUMES
According to this law, when gases react together, under similar conditions of temperature and pressure then the ratio in which their moles react is same as the ratio in which their volumes.
For example consider a combination between hydrogen gas and chlorine gas:
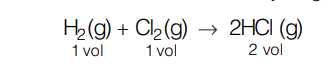
One mole of H2 will react with 1 mole of Cl2 to give 2 moles of HCl, so we can say that at the same pressure and temperature one volume of hydrogen and one volume of chlorine always combine to form two volumes of HCl gas.
The ratio between the volumes of the reactants and products in this reaction is simple, that is 1 : 1 : 2. Hence, it illustrates law of combining volumes.
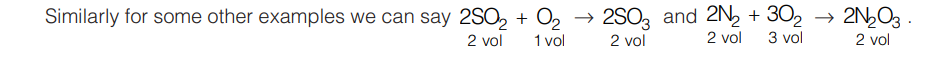
SECTION 7 : CONCEPT OF LIMITING REACTANT
In a reaction, reactants react in a fixed ratio of their moles, which are represented by the stoichiometric coefficients in the balanced chemical equation. If we consider 2H2 + O2 → 2H2O, then we can say that 2 moles of H2 react with 1 mole of O2 to give 2 moles of water.
However, in most industrial operations, exact proportions may not be present, in such a case one of the reactants gets completely consumed during the course of the reaction while the other remains unreacted as it was present in larger quantities. The reactant that gets completely consumed is called the Limiting Reactant and the one which is left out is called the Excess Reactant.
To explain this better, let us consider the reaction given above, 2H2 + O2 → 2H2O. If we have 3 moles of H2 and 0.5 moles of O2 in this process then :
- We know that 1 mole of O2 reacts with 2 moles of H2 so, 0.5 moles will react with 1 mole H2.
- So, in other words, O2 finishes off completely, whereas 3 – 1 = 2 moles of H2 will be left unreacted.
- As per our definitions, O2 will be called the limiting reactant and H2 will be called the excess reactant here.
Also learn, that the product yield is always estimated using the limiting reactant, so if 1 mole O2 gives 2 moles of water as per the equation, then 0.5 moles O2 will give 1 mole of water or 18 g water.
In order to estimate the limiting reactant, calculate the value of Moles Given/Stoichiometric Coefficient for the given reactants. The one having the smallest value of this ratio will be the limiting reactant.