Stoichiometry
SECTION 1 : CONCEPT OF EQUIVALENT MASS
- CLASSICAL DEFINITION
It is defined as the number of parts by weight of a substance that combines with or displaces directly or indirectly 1.008 parts by weight of hydrogen or 8.0 parts of oxygen or 35.5 parts of chlorine. Atomic mass and molecular mass of elements and compounds are always constant but equivalent mass may change. A compound may have different equivalent mass in different reactions.

SECTION 2: n-FACTOR CALCULATION
Case-I : Acids & Bases : Acids are the species which furnish H+ ions when dissolved in a solvent. For acids, n factor is defined as the number of H+ ions replaced by 1 mole of acid in a reaction. Note that then factor for acid is not equal to its basicity; i.e., the number of moles of replaceable H+ atoms present in one mole of acid.
Bases are the species, which furnish OH– ions when dissolved in a solvent. For bases, n-factor is defined as the number defined as the number of OH– ions replaced by 1 mole of base in a reaction. Note that n factor is not equal to its acidity, i.e., the number of moles of replaceable OH– ions present in 1 mole of base. For instance,
| n-factor for HCl | 1 | n-factor of NaOH | 1 |
| n-factor for HNO3 | 1 | n-factor of NH4OH | 1 |
| n-factor for H2SO4 | 1 or 2 | n-factor of Zn(OH)2 | 1 or 2 |
| n-factor for H3PO4 | 1 or 2 or 3 | n-factor of Ca(OH)2 | 1 or 2 |
| n-factor for H3PO3 | 1 or 2 | n-factor of Al(OH)3 | 1 or 2 or 3 |
| n-factor for H3PO2 | 1 | n-factor of Ba(OH)2 | 1 or 2 |
| n-factor for H3BO3 | 1 | n-factor of CsOH | 1 |
Case-II : If the salt involved in a reaction does not undergo oxidation state then the n factor for such salts is defined as the total cationic or total anionic charge present in 1 mole of the salt or simply the LCM of the charges on the cation and anion. For instance in the reaction, Na3PO4 + BaCl2 NaCl + Ba3(PO4)2. For instance, in Na3PO4 the LCM of charge on Na+ and is 3 and PO43– so is the total positive charge on 3Na+ ions. Same way, if we wish to find the n-factor for Ba3(PO4)2 then, it will be given as the LCM of charges on Ba2+ and PO43– that is 6.
Case-III : n-Factor in Redox Reactions : If an element in a compound undergoes a change in oxidation state then we must look at the change in oxidation state and use the under-mentioned formula for all redox changes :
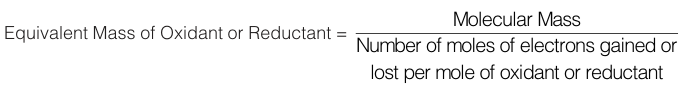
For example, let us calculate the n factor KMnO4 for the given chemical change.
![]()
In this reaction, oxidation state of Mn changes from +7 to +2. Thus, KMnO4 is acting as oxidising agent, since it is reduced.
∴ n-factor of KMnO4 = 5 in acidic media
Similarly,

It can be seen that in all the above chemical changes, KMnO4 is acting as oxidising agent, yet its n-factor is not same in all reactions. Thus, the n-factor of a compound is not fixed, it depends on the type and the extent of reaction it undergoes.
SECTION 3 : EXPRESSING CONCENTRATION OF SOLUTIONS
Solution is a homogenous mixture of two or more components in which intermingling particles are of atomic or molecular dimensions. A solution consists of a dissolved substance known as solute and the substance in which the solute is dissolved is known as solvent. The concentration of a solute means the quantity of solute dissolved per unit volume of solution, or per unit quantity of solvent.
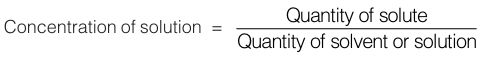
NOTE : While discussing various methods for expressing concentration, we have taken solute as B dissolved in solvent A and WB as grams of solute and WA as grams of solvent.
Molarity (M) is expressed as moles of solute contained in one litre of solution or it is also taken as millimoles of solute in 1000 cc (ml) of solution. It is denoted by M.
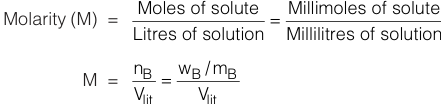
Molarity (M) is expressed as number of moles of solute dissolved in 1000 gms (1 Kg) of solvent. It is denoted by m.
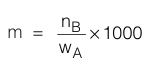
Normality (N) is expressed as the number of gram equivalents (gm eq) of solute contained in one litre of solution or it can also be taken as number of milliequivalents (meq) in 1000 cc (ml) of solution. It is denoted by N.

Strength of a solution is generally expressed as grams of solute in one litre of solution.

Diluting a Solution: Whenever a given solution of known concentration, i.e., normality and molarity (known as standard solution) is diluted by adding more of solvent, the number of millimoles (or millequivalents) of solutes remain unchanged. The concentration of solution however changes.
In such cases, M1V1 = M2V2 (M1 & V1a re molarity and volume of original solution
(M2 and V2 are molarity and volume of diluted solution)
Mass fraction is the fractional part of a component that is contributed by it to the total mass of solution.
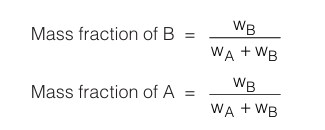
Mole fraction is the fractional part of the moles that is contributed by each component to the total number of moles that comprises the solution. In containing nA moles of solvent and nB moles of solute :
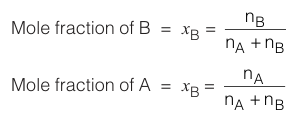
Volume Fraction
It is volume of solute over the total volume of solution.
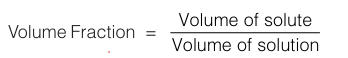
(Remember that fractions of all types always add up to give unity)
Parts per Million (ppm) and Parts per Billion (ppb)
This concentration term defines the number of milligrams of solute present per kilogram of solvent for the case of parts per million. Usually it is employed in those solutions where the solute with respect to the solution is very less.

SECTION 4 : TITRATIONS
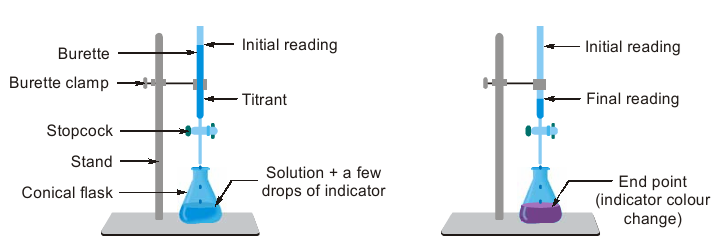
Titration is an experiment that is done to determine the unknown concentration of a substance. The substance whose concentration is known is called the Titrant or titrator. A standard solution of the titrant is prepared and added to the burette. The Titrand or Analyte is the substance that is measured in a fixed volume with the help of a pipette and placed in a conical flask (see diagram below).
As expressed in the diagram below, H2SO4 of unknown concentration is placed in a conical flask as an analyte. Few drops of phenolphthalein indicator as added to it. The solution will remain to be colourless as phenolphthalein will show pink colour in base media (pH 8.0 to 10.0).
NaOH of known concentration is placed in the burette and slowly added to H2SO4 till a faint pink colour is noted. This is the end point of the titratione at which gram equivalents of both NaOH and H2SO4 are equal.
Titrations can belong to the following categories :
Simple Titrations :
A known volume of the solution of unknown concentration is taken in a flask and required reagents are added to it. The solution of known concentration is added from the burette in the solution of unknown concentration till the latter reacts completely. This process is called titration. At the end point (equivalence point) the equivalents or milliequivalents of the two reacting substances are equal.
Volume of solution (A) = VA litres
Normality of solution (A) = NA
Equivalents of substance (A) = NAVA
Similarly, equivalents of substance (B) = NBVB
At the equivalence point (end point) the equivalents (not the moles) of the two substance are equal.
NAVA (litre) = NB x VB (litre)
Redox Titrations
In a redox titration, an oxidant is estimated by adding reductant or vice versa.
For example, Fe2+ ions can be estimated by titration against acidified KMnO4 solution when Fe2+ ions are oxidised to Fe3+ ions and KMnO4 is reduced to Mn2+ in the presence of acidic medium. KMnO4 functions as self indicator as its purple colour is discharged at the equivalence point.
MnO–4 + 8H+ + 5e– → Mn2+ + 4H2O
Fe2+ → Fe3+ + e– ] 5
MnO–4 (purple) + 8H+ + 5Fe2+ → Mn2+ + 5Fe3+ + 4H2O
(n = 5) (n = 1) Colourless.