Carbohydrates
Carbohydrates are polyhydroxy (having many – OH groups) aldehydes or ketones and their condensation products. Aldehyde group (–CHO) is generally terminal while ketone group (> C = O) is commonly sub-terminal.
Many, but not all, carbohydrates have the empirical formula of Cn(H2O)n or (CH2O)n, (where the value of integer n is 3–7 for monomers); some also contain nitrogen, phosphorus, or sulfur.
Types : Carbohydrates are of three types:
- Monosaccharides.
- Oligosaccharides.
- Polysaccharides.
A. Monosaccharides
They are simple reducing polyhydroxy aldehyde or ketone sugars or carbohydrate monomers which cannot be hydrolysed further into smaller subunits. These are generally colourless, crystalline and mostly sweet to taste.
General formula CnH2nOn or (CH2O)n. Here n = 3 to 7.
Types of Monosaccharides
Depending upon the number of carbon atoms present, the monosaccharides are of five types, each with its further aldose and ketose forms.
| Monosaccharides | 1. Trioses | 2.Tetroses | 3.Pentoses | 4.Hexoses | 5. Heptoses |
|---|---|---|---|---|---|
| A. Aldose | Glyceraldehydes | Erythrose, Threose, | Ribose, Deoxyribose, Xylose, Arabinose | Glucose, Galactose, Mannose | Glucoheptose, Galactoheptose |
| B. Ketose | Dihydroxy acetone | Erythrulose | Ribulose | Fructose | Sedoheptulose |
B. Oligosaccharides
They are small-sized polymers of monosaccharides having 2-6 simple sugars, occasionally upto 9–10, formed by condensation of two or more monosaccharides.
Depending upon the number of monosaccharides residues present, oligosaccharides are called disaccharides, trysccharides, tetrasaccharides, pentasaccharides and hexasaccharides.
- Disaccharides: Composed of two monosaccharides residues.
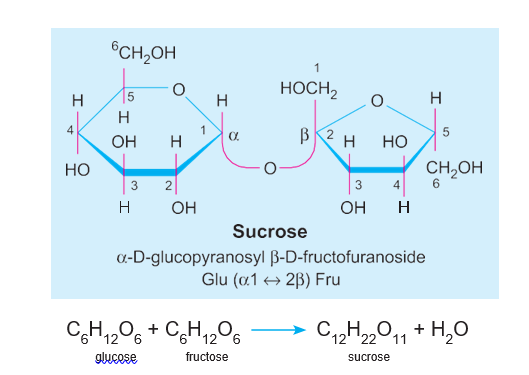
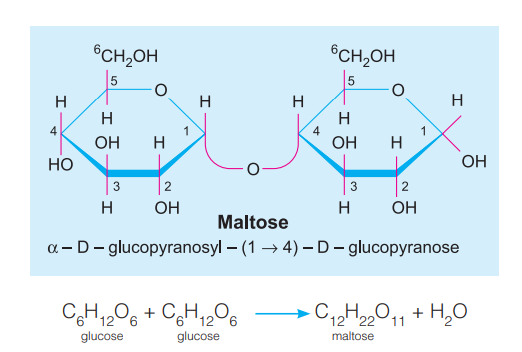
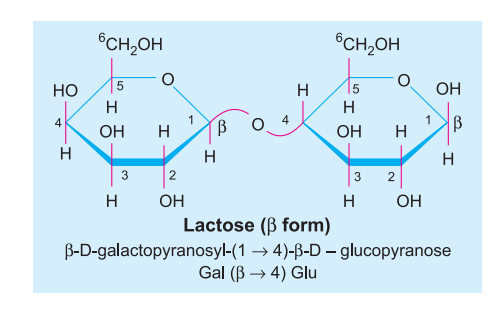
E.g. Sucrose (glucose + fructose), Lactose (glucose + galactose), Maltose (glucose + glucose).
C. Polysaccharides
Polysaccharides are complex carbohydrates, which are formed by polymerisation of more than ten but generally very large number of units called monosaccharides. Polysaccharides are also called, glycans (Gk. glykys-sweet), because of their formation from saccharides. Though formed from saccharides, polysaccharides are not sweet to taste.
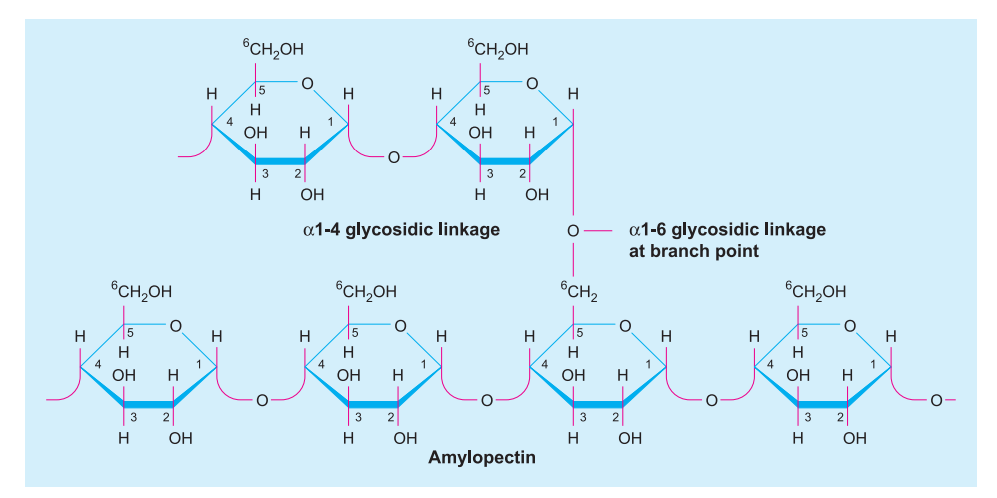
- Starch: A molecule of starch consists of two components, an inner core of amylose and an outer coat of amylopectin. The two components of starch differ in their solubility and reaction to iodine. Amylose gives blue-black colour with iodine solution (I + KI solution) while amylopectin develops red-violet colour.
Amylose is a straight but helically coiled chain of D-glucose residues (pyranose form) attached by α(1 → 4). Amylopectin is the outer branched part of starch molecule having short chains of about 25-30 glucose units linked by α(1 → 4) bonds but have branched joined by α(1 → 6) linkage.
- Glycogen (Animal starch): It is glycosan homo polysaccharide which is the major reserve food of animals, fungi and some bacteria.
It is composed of α-D-glucosopyranose units. In the straight part of chain, glucose residues are attached by α(1 → 4) linkage while in the region of branching carbon 6 of straight chain glucose residue is linked to carbon atom 1 of the side chain glucose unit. It is called α(1 → 6) linkage.
- Cellulose
Cellulose is fibrous glucosan homopolysaccharides, structural polysaccharide of plant cell walls, walls of some primitive fungi, some protists. It is the most abundant molecule of earth. Cellulose molecules are long chain polymers of 6,000–10,000 β-glucose units. They are unbranched straight and linear. The adjacent β-glucose units are joined by β(1 → 4) linkages.
- Chitin
Chitin is the second most abundant organic material.
It is structural homo polysaccharides, found in fungal walls (fungus cellulose), as chitin in the exoskeleton of arthropods. It is insoluble in water and a number of solvent.