Proteins
Proteins are polymers or heteropolymers of amino acids (Fisher and Hofmeister). Collagen is the most abundant protein in the animal world. RuBisCO is the most abundant protein in the whole biosphere. Property of protein depends (i) on sequence of amino acid and (ii) configuration of protein molecules
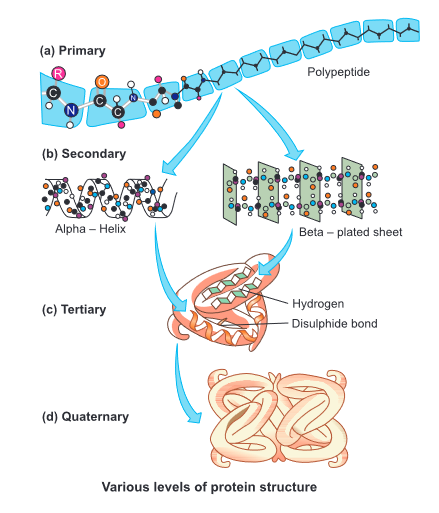
Structure of Proteins
Primary structure (1°): A polypeptide is formed of a linear row of amino acids joined by peptide bonds. Primary structure does not give any information about the functional properties of the protein, but describes the sequence of amino acid.
The left end represented by the first amino acid and the right end represented by the last amino acid. The first amino acid is also called as N-terminal amino acid. The last amino acid is called the C terminal amino acid.
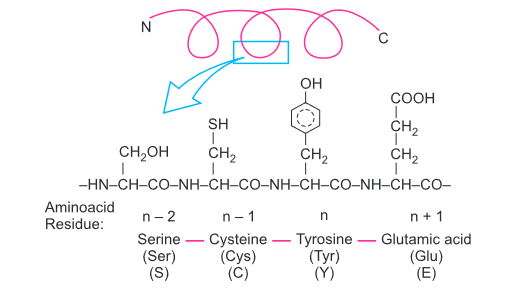
Primary structure of a portion of a hypothetical protein. N and C refer to the two terminal of every protein. Single letter codes and three letter abbreviations for amino acids are also indicated.
Secondary structure (2°): In this hydrogen bonds are also present along with peptide bonds.
Secondary structure is of three types: α-helix and, β-pleated ,Collagen helix
Tertiary structure (3°): The polypeptide chains are folded variously in tertiary structure (3°). It is bending and folding of 2° structure of polypeptide (α or β) in such a way as to form a compact structure with functional sites being established over its surface by coming together of polar regions of specific amino acids. The functional 3-dimensional structure of protein is also called native state which possesses specific areas (active sites)for its activity. Every type of protein is different from others due to specificity of such areas.
Five types of bonds are involved in forming tertiary structure of proteins. They are Vander Walls interactions, ionic bonds, hydrogen bonds, disulphide bonds and hydrophobic interactions. Some of these bonds can easily be broken by high temperature, high energy radiations, soaps, detergents, acids, alcohols and salts of heavy metals. It results in loss of three-dimensional conformation of the protein molecules. The process is called denaturation. Examples include most of the enzymes of human body like Myoglobin, Salivary amylase etc.
Quarternary structure is present in case of multimeric or oligomeric proteins. Here the different polypeptide chains interact and get oriented with respect to each other in a specific manner. Examples-
Haemoglobin has 4 polypeptide chains (2 α chains & 2 β chains). Insulin consists of 2 polypeptide chains (1 α chain & 1 β chain).